 Epitel raises $12.5M for wearable seizure detection system (MobiHealth News):
Epitel raises $12.5M for wearable seizure detection system (MobiHealth News):
Epitel, maker of a wearable electroencephalogram (EEG) system for seizure detection, announced Wednesday it had scored $12.5 million in Series A funding.
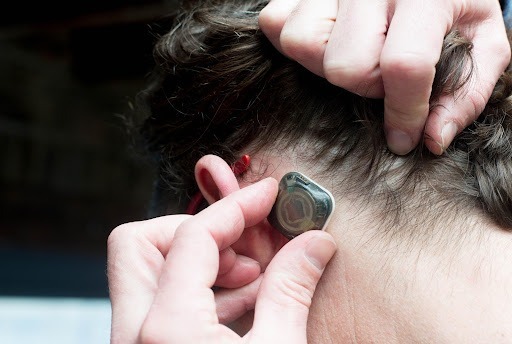
The round was led by Catalyst Health Ventures (CHV) and Genoa Ventures, with participation from Dexcom, OSF Ventures, Wavemaker 360, MedMountain Ventures and Salt Lake City Angels … The company’s first product, the REMI system, consists of a wearable, wireless EEG sensor that’s applied below the hairline, and software for providers to review data and monitor for seizures. The sensor can continuously monitor patients for 48 hours.
The platform received FDA 510(k) clearance for in-hospital use in March 2021, but Epitel plans to expand into ambulatory and at-home care in the future…
Another company focused on seizure detection is Zeit, which announced in November it had raised $2 million in seed funding. The company makes a headband device that aims to alert patients to signs of a stroke or seizure. Zeit said the seed investment would go toward hiring new team members and funding a submission to the FDA.
Remote monitoring company Empatica makes an FDA-cleared wrist-worn device for people with epilepsy to alert users and their caregivers of convulsive seizures.
The Announcement:
Epitel Secures $12.5 Million Series A Financing for Wearable, Wireless EEG Monitoring System (press release):
Epitel, Inc., a digital health company developing a wearable, wireless EEG monitoring platform for seizure detection, announced today the closing of a $12.5 million Series A financing for initial pilot commercialization and further development of its proprietary platform. Epitel received FDA clearance for its first product, a wireless and wearable EEG (brain wave monitor) sensor, and remote access software known as REMI® for use within hospital emergency rooms and critical care units. REMI first received clearance from the U.S. Food & Drug Administration in 2021.
Two-thirds of the U.S. population lack ready access to EEGs and most emergency departments lack the capability to adequately monitor EEG … REMI, Epitel’s first FDA-cleared product solves this problem with wearable, wireless sensors that can be rapidly and easily applied by a nurse or hospital technician. EEG data is then immediately connected to a cloud-based software platform available to neurologists to review and monitor for seizures at any time from any location. Because the Epitel System is wearable and wireless, it can continue to monitor the patient continuously for 48 hours during their hospital journey.
“It is time that EEGs for the brain become as accessible as EKGs for the heart to patients throughout the country. For too long essential neurological services have been inaccessible to large parts of our population,” said Dr. Chaudery, Principal with Genoa Ventures.
News in Context:
- Study: Wearable sensors and machine learning may well (one day) help detect a broad range of epileptic seizures
- Special Issue: Seizure detection and mobile health devices in epilepsy
- Study: Brain scans mapping language and memory areas can help guide epilepsy-related surgeries
The post Wearable EEG monitoring start-up Epitel raises $12.5M to market seizure detection system appeared first on SharpBrains.





















